
- Видео 283
- Просмотров 31 227 933
Leah4sci
США
Добавлен 3 сен 2011
Complex organic chemistry concepts and mechanisms made easy through simple tutorials, step by step mechanisms, and detailed explanations every step of the way.
Help spread the word - post a flyer in YOUR orgo classroom.
Print Flyer: leah4sci.com/flyer
Help spread the word - post a flyer in YOUR orgo classroom.
Print Flyer: leah4sci.com/flyer
Chain Elongation via Alkylation of Terminal Alkynes
Chain Elongation via Alkylation of Terminal Alkynes
Просмотров: 3 314
Видео
Choosing Between SN1 SN2 E1 E2 Reactions
Просмотров 31 тыс.7 месяцев назад
Choosing Between SN1 SN2 E1 E2 Reactions
Hydride Shift vs Methyl Shift - Carbocation Rearrangement
Просмотров 11 тыс.8 месяцев назад
Hydride Shift vs Methyl Shift - Carbocation Rearrangement
Enantiomers, Diastereomers and Meso Compounds: Multiple Chiral Centers
Просмотров 20 тыс.8 месяцев назад
Enantiomers, Diastereomers and Meso Compounds: Multiple Chiral Centers
Organometallic Reagents and Reactions - Grignard, Gilman, Organolithium
Просмотров 22 тыс.Год назад
Organometallic Reagents and Reactions - Grignard, Gilman, Organolithium
Alkyne Hydroboration Oxidation Reaction and Mechanism
Просмотров 22 тыс.Год назад
Alkyne Hydroboration Oxidation Reaction and Mechanism
Organic Chemistry Professors Expect You Remember This From General Chemistry
Просмотров 17 тыс.Год назад
Organic Chemistry Professors Expect You Remember This From General Chemistry
Grignard to Alcohol Synthesis Shortcuts - Aldehyde, Ketone, Ester
Просмотров 32 тыс.2 года назад
Grignard to Alcohol Synthesis Shortcuts - Aldehyde, Ketone, Ester
E2 Reaction Coordinate Energy Diagram
Просмотров 7 тыс.2 года назад
E2 Reaction Coordinate Energy Diagram
Diels Alder Reaction Stereochemistry and Endo vs Exo Products
Просмотров 54 тыс.2 года назад
Diels Alder Reaction Stereochemistry and Endo vs Exo Products
Grignard Attacks Nitrile to Form Ketone - Reaction and Mechanism
Просмотров 27 тыс.2 года назад
Grignard Attacks Nitrile to Form Ketone - Reaction and Mechanism
TMS Alcohol Protecting Group Using Silyl Ether
Просмотров 13 тыс.2 года назад
TMS Alcohol Protecting Group Using Silyl Ether
Quick Organic Chemistry 1 Reactions Review - Alkene Alkyne Radical Substitution Elimination
Просмотров 21 тыс.2 года назад
Quick Organic Chemistry 1 Reactions Review - Alkene Alkyne Radical Substitution Elimination
E1 Reaction Coordinate Energy Diagram
Просмотров 9 тыс.2 года назад
E1 Reaction Coordinate Energy Diagram
Alkyne Oxymercuration Demercuration Reaction and Mechanism
Просмотров 21 тыс.2 года назад
Alkyne Oxymercuration Demercuration Reaction and Mechanism
Cyclopropanation of Alkenes Carbene via Haloform and Simmons Smith Reactions
Просмотров 25 тыс.2 года назад
Cyclopropanation of Alkenes Carbene via Haloform and Simmons Smith Reactions
Anti-Markovnikov Radical Halogenation of Alkenes
Просмотров 16 тыс.2 года назад
Anti-Markovnikov Radical Halogenation of Alkenes
Initiation, Propagation, Termination - 3 Steps of Radical Reactions
Просмотров 157 тыс.2 года назад
Initiation, Propagation, Termination - 3 Steps of Radical Reactions
HOMO and LUMO Molecular Orbitals for Conjugated Systems by Leah4sci
Просмотров 125 тыс.3 года назад
HOMO and LUMO Molecular Orbitals for Conjugated Systems by Leah4sci
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
Просмотров 127 тыс.3 года назад
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
IUPAC Naming Practice - Nomenclature for alkanes, dienes, alcohols and more
Просмотров 112 тыс.3 года назад
IUPAC Naming Practice - Nomenclature for alkanes, dienes, alcohols and more
Functional Groups Practice for Organic Chemistry
Просмотров 64 тыс.3 года назад
Functional Groups Practice for Organic Chemistry
Lewis Structure, Shape, Hybridization and Polarity Practice (Organic Chemistry)
Просмотров 26 тыс.3 года назад
Lewis Structure, Shape, Hybridization and Polarity Practice (Organic Chemistry)
Grignard Reagent, Reaction, Mechanism and Shortcut
Просмотров 120 тыс.4 года назад
Grignard Reagent, Reaction, Mechanism and Shortcut
H-NMR Predicting Molecular Structure Using Formula + Graph
Просмотров 310 тыс.4 года назад
H-NMR Predicting Molecular Structure Using Formula Graph
Alkyne Acid Catalyzed Hydration Reaction and Mechanism
Просмотров 40 тыс.4 года назад
Alkyne Acid Catalyzed Hydration Reaction and Mechanism
Markovnikov’s Rule vs Anti-Markovnikov in Alkene Addition Reactions
Просмотров 261 тыс.4 года назад
Markovnikov’s Rule vs Anti-Markovnikov in Alkene Addition Reactions
Thank You for 100k Subscribers! Unboxing YouTube Silver Play Button Award
Просмотров 8 тыс.4 года назад
Thank You for 100k Subscribers! Unboxing RUclips Silver Play Button Award
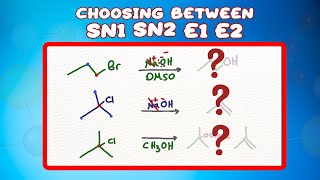








Please is it intentional that the 1 and 2 for the SN reaction is superscript and for the Elimination subscript
I tell students that when electrons are spin-paired, they are in a stable relationship. They can get excited into an "unhappy state". This are great videos for our students to learn bonding!
Very thorough yet easy to understand, it will help guid my allied health srudents through orgranic chemistry
you will definitely go to heaven. thank you so much . saved me :)
Aww thank you, so glad I could help!
Taking Orgo 1 AND 2 combined in 7 weeks. *Crying*
OUCH! It's vital that you get ahead as much as possible and go in each day caught up (and reading ahead) so that you can optimize every moment of your class time.
is there Carboxylic Acid reactions mechanisims
Please make a video on syn and anti addition🙏
Thanks for the recommendation. I post free videos as I have time and am always looking for suggestions.
thank youuuu❤✨
You are so welcome!
do you have a series for carboxylic acid reactions?
You can find what I have so far on my free syllabus guide: leah4sci.com/syllabus
Hello, could you please explain to me the meaning of some substances written on reaction arrows, such as Zn-Hg, Zn(Hg), Zn-H2O, EtOH/H2O, BH3·THF? What do the parentheses (), dot (.), dash (-), and slash (/) signify in these cases?
4:32 Can we say that the mechanism is SNA/E?
thank you
You're welcome!
Ur helping me to get out from depression . Thanku so so much❤ Luv u
I'm so happy to be a help for you during such a tough time. Sending hugs.
i loveee your videos, they are by far the best and most easiest to understand. thank you for all your help! also, is there any way you could possibly make videos explaining over nucleophilics/electrophilics & gibbs free energy/thermodynamics/kinetics?
you're a life saver
Happy to throw you a line!
Thanks ma`am.....I love your videos
You're welcome, glad you love them!
This the best leacture on Chesmistry I have ever attained. Thank you so much
Wow, thanks so much! Glad I can help
thank you, you helped me a lot :)
You are welcome!
I pretty sure Cyclooctatetraene is not planar and is kind of in a boat-like structure? Therefore, it doesn't count as aromatic. Other than that, awesome video! Thanks a lot ^_^
wow…. your videos are amazing thank you so much, this saved me from my 2 year brain drain, subscribed and liked thank you
Awesome, thank you! I'm so glad I was able to help you refresh what you had learned!
Wow this is what I need to understand chemistry you are the best I've never seen someone who explains like this in my entire life,can I stay with you plz 🙂
You are so very welcome and yes, I'm happy to be here with you on your journey. Check out more of my free resources here: leah4sci.com/syllabus
Hii i am having problem with cyclo free radical compund with a pie bond resonance can you please tell how can solve it . Btw u are a great teacher ❤ loved your content
Thanks!
Thank you so very much, I appreciate it!
Thank u mam i was crying that i didnt understand this in coaching U simplified it a lot
I'm sorry to hear this topic had you in tears, but I'm glad I was able to help clear things up for you!
Aah! Masha Allah ...... Superb
Thanks, glad you liked it!
4:37 why not the elctron pi on C=O attack H+ why it has to be the electron on Oxy attack H+ I am confuse in this. Is it have a possible mechanism that the pi electron attack H+ not the Oxy electron?
Good question! First, the lone pair electrons are more free to move and attack than electrons that are tied into a pi bond. Second, if the pi bond were to attack, we would be left with either an oxygen or carbon atom that does not follow the octet rule. Oxygen and carbon are both too small to be exceptions to the octet rule.
@@Leah4sci I agree with point 1. But when you say, 'if the pi bond were to attack, we would be left with either an oxygen or carbon atom that does not follow the octet rule,' do you mean that if the pi bond attacks, it could either create a carbocation or a CH-O+ (I'm not sure how to pronounce a positively charged oxygen)? I don't think these two cases violate the octet rule because they result in oxygen and carbon having empty orbitals, and there are many reaction mechanisms where carbocations are formed, such as in SN1 reactions. However, I've never seen a positively charged oxygen because the C=O bond is more polarized towards oxygen, so only oxygen forms bonds with pi electrons with electrophiles like H+. Yesterday, I asked my teacher why he wrote that the pi bond attacks H+. His answer was, "when you become proficient in reaction mechanisms, you can omit some steps in the mechanism. If you want to write that the lone pair on oxygen attacks H+ and then the pi electrons become the lone pair on oxygen, that's also correct."
Thank you from Jordan ❤
You're very welcome!
How can the above the branch chain is gave a priority than alkyne with the same opportunity to choice
I think you've spotted the error in this video! Nice job. :) Notice the error is mentioned in the title of the video as well as the description. My note is as follows: Note: The molecule at 3:00 is named incorrectly and should be numbered in the opposite direction. The correct name for this structure would be 6-methyl-2-heptyne.
my bachelor degree in pharmacy should be given to you
Thank you, but you keep it ;)
For real
👍 👍
Glad you liked it!
Hi Leah, thanks for the video. You meant to say "place the pi bond between C1 & C2" because in your video, you said "place the pi bond between C2 & C3."
I'm having a hard time finding the audio error that you mention. At 8:46, I say that we have the pi bond between carbon 1 and 2. If I mention it otherwise for that example at any point in the video, please disregard. :)
Shouldn't the carbon on the structure with 4 resonances be sp3 rather than sp2? Around the 10 minute mark?
The sp2 carbon atom on the screen at 10:00 is labeled correctly. It has 3 bonds around it (1 double bond and 2 single bonds) and 0 lone pairs. That makes it an sp2 hybridized carbon. For an in depth look at sp3, sp2 and sp hybridization, make sure to view my tutorial on my website at Leah4sci.com/Hybrid
Hi Can i name the secbutyl -isobutyl
No, secbutyl and isobutyl are two different substituents. You can see them drawn separately on the screen around 7:00. Each group has a different branching pattern, and therefore should be named differently. For more Naming practice and resources, make sure to see my Naming tutorial series and cheat sheet at Leah4sci.com/Naming
Thank you for the videos. I’m getting much much help from you! Btw I just have q question. 10:45 On the 4 th resonance molecule, Why the black electrons stop moving to the right side and the red electrons going back to the oxygen? Why it doesn’t have 5 resonance?
Great question. Doing so recreates what we see in Resonance Structure #1, where we have a negatively charged oxygen with alternating pi bonds throughout the ring. The pi bonds will simply be shifted one space over from where they started originally. Most professors will consider this the same molecule as #1. But drawing it does not necessarily make you wrong either!
Thank you very much. Your explanation is very clear🎉
Glad it was helpful!
Thank you ❤ from Algeria 🇩🇿🫂
You're welcome!
But sulfur crossed the octet rule, it has total 12 electrons now 😢
Yes, and sulfur is an exception to the octet rule in this case. It is a large enough atom (with available d orbitals) that it can hold up to 12 electrons around it.
To make it more simple, is it okay to swap only once, and think it is the opposite direction? Ex. I swapped once, and became S. Then it is R!
Yes! That method works fine too. Use what works for you!
I wrote my final exam in organic chemistry just wanted to say thank you for making it bearable
So happy that I was able to help you!
God bless you and grants you more wisdom
Thank you!
You made it really good video, I don’t like this chapter but with our video I’m starting to understand 🌷, I still have a question : when an acid is weak (and therefore stable) it still reacts with au to give its conjugate base And even if it reacts with water because even if it is weak it still gives its H+ Why does its conjugate base, which is strong and therefore unstable, not re-react with an H+ to regain its stability (why does it continue to exist) Why is there an equilibrium when a weak acid reacts with water whereas normally its conjugate base which will be unstable will have to reform the acid
Love ur vids omg
Thank you!
I love my professor for the rest of the topics... but he wasnt so good at explaining the reactiojs part 😢 Thank❤ you
So happy to help fill the gap and it's great that overall you have a good professor.
Orgo 2
Hope your class is going well
Thanks!
You're welcome!
1:15 why Br is attacking back.
Br is an electronegative atom, when the pi bond attacks, the carbon no longer holding the pi bond is now left deficient and therefor positive. The electronegative Br is attracted to it and therefor attacks
@@Leah4sci Thank you..
Excellent ❤❤❤❤❤
Thanks!
thank you leah you've made me understand what i've been struggling with my exam is tomorrow
So glad I could help!
Even after 3 years , this video proves to be very very useful ❤
So glad to help!